By Lüder Deecke
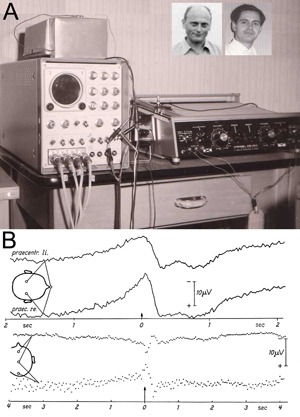
Figure 1: Original experimental setup (A) and first results (B) in Freiburg, Germany, at the University Hospital of Neurology with Clinical Neurophysiology, Hansastr. 9a, Freiburg, known popularly as “Neurophys.”
A: The centerpiece of the laboratory — the Mnemotron CAT Computer (Computer of Average Transients) 400 B and Mosely Autograf (XY-Plotter). Prof Richard Jung, head of the hospital, had been offered a post at the Max Planck Institute in Munich. For rejecting this, his home University of Freiburg gave him the CAT Averager as a present for staying. The two inserted photos show Hans Helmut Kornhuber on the left (age 36 in 1964) and Lüder Deecke on the right (age 26 in 1964).
B: Above: Changes in brain potentials with voluntary movements of the left hand based on unipolar recordings of the precentral region versus the nose (Subject G.F., average of 512 movements). Onset of movement at the arrow (0 sec) – left of the arrow brain activity prior to movement onset — right of the arrow brain activity after the onset of movement. Note the negative potential at readiness and the positive potential after the action. The graph shows higher amplitudes over the contralateral (right) hemisphere. Negative polarity is up.
Below: The bipolar serial recording with sagittal electrodes with voluntary movements of the right hand (average from 400 movements. Subject B.C.). The graph shows phase reversal around the precentral electrode: The premotor negativity and the postmotor positivity are strongest over the central region. In the fronto-precentral recording negativity of the precentral electrode is down, in the pre-centro-occipital recording negativity of the precentral electrode is up.
A special session inaugurated and chaired by Mark Hallett, Bethesda, Maryland, at the International Congress of Clinical Neurophysiology (ICCN2014) in March 2014 in Berlin celebrated the 50th anniversary of the Bereitschaftspotential. The session included lectures by Lüder Deecke, Vienna: “Experiments Into Readiness for Action — Bereitschaftspotential;” Hiroshi Shibasaki, Akio Ikeda, Kyoto, Japan: “Generator Mechanisms of BP and Its Clinical Application;” Gert Pfurtscheller, Graz, Austria: “Movement-Related Desynchronization and Resting State Sensorimotor Networks;” and Ross Cunnington, Brisbane, Australia: “Concurrent fMRI-EEG and the Bereitschafts-BOLD-Effect.” The session was well accepted.
In this paper, I would like to give an outline of the history of the Bereitschaftspotential and a selection of the main research results of our experiments into readiness for action.
The History of the Bereitschaftspotential
In 1964, my mentor Hans Helmut Kornhuber (1928-2009) and I discovered the readiness potential (Kornhuber and Deecke, 1964). We submitted the full paper in the same year. It was published in the first 1965 issue of “Pflügers Archiv” (Kornhuber and Deecke, 1965).
We described a novel method, reverse averaging, for recording brain electrical activity prior to voluntary movement in humans by noninvasive means and presented the first fundamental results obtained with this method. We found that a negative electrical cortical potential consistently preceded human voluntary movement and named it the Bereitschaftspotential (BP) or readiness potential. (See Figure 1B.)
The BP is the electrophysiological sign of planning, preparation and initiation of volitional acts. How did the idea come up to record brain potentials preceding human voluntary movements in the EEG? It began on a Saturday in May 1964 when Kornhuber invited his doctoral student L.D. for lunch into the ‘Gasthof zum Schwanen’ at the foot of the Schlossberg hill in Freiburg, Germany (near the Black Forest).
We sat in the beautiful garden and discussed our frustration with the fact that the brain was investigated — at that time — only as a responsive apparatus, i.e. as a mere reacting system. Neurophysiologists were engaged worldwide only in what was called “the responsive brain” (later culminating in a book with this title by McCallum & Knott, 1976). We felt that it would be far more exciting to investigate what is going on in our brain before we make a voluntary movement. No sooner said than done.
We went back to the lab and started immediately planning the experiment. However, we soon ran into an important problem: Brain potentials in the EEG are the result of averaging. For us to get the results we needed, the averaging process must be triggered by the movement or action itself. But how can you trigger on an event that comes as unpredictably and spontaneously as a human voluntary movement?
It was Hans Kornhuber, who found the solution: We would store the EEG on magnetic tape along with the electromyogram (EMG) of the movements and then play the tape backward in the time-reversed direction from the present back to the past, i.e. using reverse averaging with the start of the movement as the trigger. At that time, magnetic tape recorders only had a high-speed rewind, and programmable computers were not yet available, so we were literally removing the tape reels from the recorder, turning them around, and placing them back on the recorder. By these means, we found a brain potential, which was electrically negative and started already 1½ to 1¼ seconds prior to the movement or action. Negativity in the brain means activity.
This method of recording of the readiness potential — Bereitschaftspotential by reverse averaging was the basis of my doctoral thesis, which I completed in 1965 at the University of Freiburg with Korn-huber as my doctoral supervisor. The experiments that led to the discovery of the Bereitschaftspotential were inspired by a positive concept of will, i.e. individual humans have, indeed, their own will and own decision-making power, so that we are capable of designing our lives largely by ourselves and use goal-oriented action to create our own future.
Kornhuber lectured on human freedom in a seminar for students of all faculties. He conducted a survey among his listeners as to who is freer: humans or chimpanzees, chimpanzees or rhesus monkeys, rhesus monkeys or cats, cats or salamanders, salamanders or spiders, and so on until down to the earthworm. The seminar participants answered the question of freedom unambiguously, namely according to the position of the animal in the evolution.
Obviously, we interpret evolution as a process making organisms freer and freer; one also can say making them more and more autonomous. Similarly, we consider an adept, e.g. Shaolin monk, who works hard on his own personality, to become more and more free. Kornhuber was of the opinion and demonstrated that one can make freedom a topic of scientific investigation. Not to deal with freedom only philosophically but also to explore it using scientific means (Kornhuber, 1978; 1984; 1987; 1988; 1992; 1993).
Immanuel Kant, the great German philosopher of the enlightenment (who coincidentally was born in the same town, Königsberg, as Kornhuber) was the first to distinguish between two fundamental aspects of freedom, namely freedom from and freedom to. Freedom to is the much more important aspect here, and this distinction was taken up and further developed by Kornhuber. The scientific breeding ground for the experiments toward the Bereitschaftspotential was thus already prepared well in advance. Rather than being a serendipitous discovery, the Bereitschaftspotential was therefore the result of a new branch of research planned by Kornhuber and myself.
The Laboratory: Original Experimental Setup in Freiburg/Germany

Figure 2: Chairman and speakers in the session “Bereitschaftspotential – 50 Years After Its Discovery” at the International Congress of Clinical Neurophysiology 2014 (ICCN2014) on March 20, 2014, in Berlin. From left to right: Gert Pfurtscheller, Graz, Austria; Ross Cunnington, Brisbane, Australia; Lüder Deecke, Vienna; Hiroshi Shibasaki, Kyoto, Japan; and Mark Hallett, Bethesda, Maryland (Chairman).
During the experiments, the subject sat in a Faraday cage for electrical shielding. The EEG was recorded using a Schwarzer-EEG Type E 502 with tube amplifiers and the EMG by a Tönnies-EMG and Stimulation Unit. Data were stored using a Telefunken four-channel magnetic tape recorder M 24-4, frequency-modulated. The tape reels were turned around for reversed averaging. The center-piece of the experimental setup is shown in Figure 1A: Mnemotron CAT Computer 400 B with Mosely Autograf. (See Figure 1: How Kornhuber and I came to the opportunity to use this then-ultra-modern device for our experiments.)
Figure 1B gives one of our first results, a slowly increasing ramp-up of negativity was recorded, which was stronger over the contralateral hemisphere. Negativity culminated at movement onset (and after the action returning to positivity), which we called the Bereitschaftspotential. In the lower set of graphs in Figure 1B, this negativity was demonstrated by a bipolar recording to show phase reversal around the precentral electrode. We called the pre-movement negativity the “Bereitschaftspotential” and in our English summary of Kornhuber and Deecke (1965) offered the English translation “readiness potential.” Somehow the tongue-twister Bereitschaftspotential was preferred and is now a German word in the English language.
We instructed our subjects to make their movements:
- at irregular intervals
- out of free will
- of their own accord
The movements we analyzed were therefore entirely self-initiated without external simuli. We dislike the term “self-paced,” suggesting regular pace, while it is so important to make the movements at irregular intervals, which make them more volitional, and regular pace makes them more automatic. At that time (1964), this was a remarkable instruction for subjects, to which I think we owe our success. And, thus, a brain potential evolved completely different from W. Grey Walter’s (1924-1971) expectance wave or CNV (both having been first published in 1964). By the way, Walter came from Bristol in 1964 to make summer vacation in the Black Forest, which was fashionable for British at the time. He visited Richard Jung, and Kornhuber and I showed Walter our first results. It was Walter who in a later publication coined the term “opisthochronic averaging” for our methodology. Our first movements under study were simple movements (rapid flexions of the forefinger).
Another methodological prerequisite is to investigate monophasic movements, i.e. that the flexed finger remains in the flexed position until the end of the analysis epoch. Using wrist extension and flexion in one flick of the hand is not good, since this employs two movements instead of one.
By comparing active movements with analogous passive ones, we aimed to show that the BP occurs prior to active movements only. To initiate passive movements, the experimenter pulled a string that was fixed to the subject’s finger and ran over a pulley, so that pulling would cause the subject’s finger to flex. Indeed, we did not detect any BP prior to such passive movements, but recorded evoked potentials after movement onset elicited by the passive movement. Post-movement onset potentials also occurred in the active state. We referred to these as “reafferent potentials” because the term “evoked potentials” should, by definition, be reserved for potentials that are elicited by external stimuli.
Citation Classics
Our first full paper (Kornhuber and Deecke, 1965) became a Citation Classic on Jan. 22, 1990. Eugene Garfield of the journal database Current Contents (CC) “awarded” this label to papers that were frequently cited. For a paper written in German, this does not occur too often. Garfield gave a translation of the German title: “Changes in Brain Potentials With Willful and Passive Movements in Humans: the Readiness Potential and Reafferent Potentials.” As part of receiving Citation Classic status, we were asked to write up how we arrived at our discovery (Citation Classic Commentary), and we gave our commentary the title: “Readiness for Movement – The Bereitschaftspotential Story” (Kornhuber HH & Deecke L (1990).
The Citation Classic Commentary was published both in CC Life Sciences and in CC Clinical Medicine. Three more of our papers became Citation Classics: No. 2 was Deecke, Scheid, Kornhuber (1969). Here we continued our investigation into the cerebral activity preceding willful movement, and also compared finger movements with arm movements. The 1963 Nobel Laureate for medicine, Sir John Eccles was interested in our work. In his 1977 book, “The Self and Its Brain,” which he co-auhored with Karl R. Popper, he wrote about our research: “There is a delightful parallel between these impressively simple experiments and the experiments of Galileo Galilei who investigated the laws of motion of the universe with metal balls on an inclined plane.”
In his previous book, “The Understanding of the Brain” (Eccles JC 1973), he wrote (page 108): “In an initial investigation by Grey Walter, the subject was trained to perform a movement after a double stimulus sequence: a conditioning, then a later indicative stimulus. An expectancy wave was observed as a negativity over the cerebral cortex before the indicative stimulus. Essentially, this wave is produced by the conditioned expectancy of the indicative stimulus and not by a voluntary movement. The problem is to have a movement executed by the subject entirely on his own volition, and yet to have accurate timing in order to average the very small potentials recorded from the surface of the skull. This has been solved by Kornhuber and his associate who used the onset of the movement to trigger a reverse computation of the potentials up to 2 seconds before the onset of the movement. The subject initiates these movements “at will” at irregular intervals of many seconds. In this way, it was possible to average 250 records of the potentials evoked at various sites over the surface of the skull, as shown by the numbers in Figure 4-3 and the corresponding traces. (Figure 4-3 is taken from Deecke, Scheid. Kornhuber [1969] and shows the comparison between finger and arm movements.) These experiments at least provide a partial answer to the question: What is happening in my brain at the time I am deciding on some motor act?”
Citation Classic No. 3, Deecke, Grözinger, Kornhuber (1976), resulted from my habilitation thesis, a requirement to become a professor at a German university. This paper in Biological Cybernetics, “Voluntary Finger Movement in Man: Cerebral Potentials and Theory,” comprises a lot of experiments performed in Ulm with many figures and a comprehensive analysis of the BP and its components. Three different brain potentials preceding voluntary rapid finger flexion were distinguished:
- Early negative activity of the Bereitschaftspotential — widespread
- Pre-motion positivity, PMP – widespread
- Motor potential (MP) – unilateral, restricted to the contralateral motor cortex.
Two years later, Citation Classic No. 4 (Deecke & Kornhuber, 1978) was published. This paper in Brain Research, “An electrical sign of participation of the mesial ‘supplementary’ motor cortex in human voluntary movements” first suggests that the early component of the BP, BP1 or BPearly is generated by the SMA and — as we found later — also by the cingulate motor area (CMA). Both being clinicians, Kornhuber and I also investigated patients and were early on able to link Parkinson’s disease (PD) with the Bereitschaftspotential. Working with patients means investigating lesion experiments made by nature, and if carefully studied, their pathological state can tell us a lot about the normal function.
Kornhuber had worked on the basal ganglia and cerebellum and found that the basal ganglia are an important component of the cortico-basal ganglia-thalamo-cortical loop. This loop was known by neurosurgeons long ago and created the prerequisites for the thalamotomies in classical stereotaxic surgery. The cortico-basal ganglia-thalamocortical loop was later confirmed and called motor loop by Alexander et al (1986), and by Mahlon DeLong (1990). Nowadays, we have to “think in loops,” and the motor loop is the key to the understanding of Parkinson’s disease. In our Citation Classic No. 4 (Deecke & Kornhuber 1978), we found differences in the BP between Parkinson’s disease patients and normals. One of the best examples of how the BP in Parkinsonian patients looks like came from an elderly woman among our subjects — she was a duchess — suffering from Parkinson´s disease who was trying hard in our BP experiment to meet our performance criteria. The main feature was that we found a high amplitude BP over the vertex but not very much of a BP in the contralateral and ipsilateral precentral leads due to her PD. Experiments were carried out in patients with bilateral Parkinsonism selected for pronounced akinesia but minimal tremor, and in age-matched healthy control subjects. The vertex maximum of the Bereitschaftspotential had previously been explained by volume conduction. It was argued that a vertex electrode collects activity from both motor cortices. In bilateral Parkinsonism, however, we see a bilateral reduction of the Bereitschaftspotential in the precentral area, whereas over other cortical areas (in particular vertex and mid-parietal), it was not significantly reduced. This publication therefore established the SMA participation in the generation of the BP, and our findings were confirmed by CD Marsden (1938-1998) (Marsden et al 1996). Barrett, Shibasaki & Neshige (1986) reported that the BP is normal in PD. But Dick et al (1989) found that the BP is abnormal in PD. Our group repeated the experiments and confirmed the findings of Dick et al 1989. Harasko, van der Meer et al 1996 cited in Lang and Deecke 1998 ibidem Figure 5 on page 235. Results: In the PD patients (N=8), the BP starts later and is initially lower in amplitude as compared to the normal controls (N=8). After the onset of movement, the amplitudes are equal and even sometimes larger in the PD patients as compared to the controls. Thus, Parkinsonian patients start later with their BP, but then keep up with the normal controls.
In May 1979, Kornhuber and I organized the MOSS V congress (one of the series of the EPIC congresses) in ReisensburgCastle, near Ulm, Germany. Afterward, we edited a book of the conference proceedings: Progress in Brain Research Vol 54 (Kornhuber & Deecke [Eds] 1980). The next conference on the Bereitschaftspotential was an international symposium in April 1988 in Vienna. which I organized in honor of Kornhuber’s 60th birthday. The proceedings also were published as a book (Deecke, Eccles, Mountcastle [Eds] 1990).
The Dispute With Libet
In October 1988, Sir John Eccles invited scientists working on the motor system to contribute to a study week in the Vatican, titled “The Principles of Design and Operation of the Brain” and a resulting book (Eccles & Creutzfeldt [Eds] 1990). I met Benjamin Libet at earlier conferences, and he also attended the Vatican Study Week. We liked each other and became friends, although not being of the same opinion regarding the freedom of human will. He worked with Wundt’s event clock and asked his subjects to remember the position of a red dot on the clock at the instant when the “conscious urge to move” occurred to him or her. Libet made a big claim in his Vatican lecture (Libet 1990) by saying that freedom is firmly linked with consciousness, the state of full awareness. He brings it to the point in his own words: “The unconscious initiation of a freely voluntary act.” Libet is correct in this statement, but his interpretation is not correct. He takes it for granted that in our unconscious or preconscious inner world there is no freedom, and thus concluded that we have free will in the control of the movement but not in its initiation. This is because the “W” in his paradigm (conscious wish) comes later than the start of the readiness potential.
Kornhuber and I, joined by the philosopher Daniel C. Dennett, are of the opinion that there are conscious and unconscious agendas in the brain, and both are important. The conscious and unconscious agendas of the brain were the subject of a workshop at the European Neurological Society (ENS) Congress 2010 in Berlin, where Dennett and Adrian Owen were the speakers. I also expressed our view in a paper in Brain Sciences (Deecke L, 2012). Libet’s paradigm is a mixture of cerebral and subjective events, and the problem lies in the subjective event (W).
It is hard to trace back split seconds, and 200 msec is very, very short. I tried the Libet paradigm myself with students, and we were not able to accurately perform in his paradigm with Wundt’s event clock position that has to be recalled retrospectively. Wundt’s event clock has been designed for sensory psychophysical experiments.
In a voluntary movement paradigm (BP-paradigm), it is like a “foreign body,” because it is an external stimulus and thus disturbs the “volitionality,” so to say, the willfullness of the movement or ac-tion. In conclusion, Kornhuber and I feel that the importance of consciousness has been underestimated by the behaviorists. It is not an epiphenomenon. If after brain injury, consciousness is regained, the lesion can (partially) be compensated, however never without consciousness. Conscious awareness is a shining light of freedom, although it should not be overestimated either.
For most of the vital functions, consciousness is not necessary, and it is not the only sign of freedom. The experiment of Libet et al (1983), which showed that the BP is not, right from the beginning, accompanied by a consciousness about the intention of movement is taken at present as the main argument for advocating a total determinism, a complete unfreedom of humans. This position is not tenable. What would be essential to study is the original planning and decision. This, however, has been completed already before the beginning of the experiment, when subjects gave their informed consent to the experimenter’s instructions. Repetitions of stereotyped simple movements are not suitable for such an investigation.
Thoughts of planning and motivation are as we all know performed in the light of consciousness. The conscious awareness to want to make a movement does occur, in investigations of the BP, about 200 ms prior to the muscle contraction — Libet’s W. This is roughly the same time span needed for a motor reaction upon an expected auditory stimulus. Although the decision to act already has been made earlier, consciousness is switched on in order to be able to make changes to the movement if necessary — changes that can go as far as not executing the action at all (Libet’s veto), and to be able to learn from the success of the movement. In both cases, the following brain areas are activated: the SMA, the pre-SMA, the anterior portion of the cingulate cortex (CMA) and a part of the motor cortex (Cunnington et al 2000), for which the basal ganglia do the groundwork (Kornhuber and Deecke 2012). However, with the self-initiated movement — but not with externally triggered movements — additionally the basal ganglia are activated before the movement (Cunnington et al, 2002). This preparatory process for the spontaneous movement, through which the readiness for movement in the SMA builds up, remains unconscious for the first 400 ms, as Libet has demonstrated.
It is not unusual for something to happen unconsciously in the brain as well as in sensory systems. In the motor system, processes that are initially conscious can become unconscious through automatiza-tion (also cf. Wu et al 2004). The switching on of consciousness shortly before the movement is a great expenditure for the brain and shows that even such “unimportant” repeated movements needed for the averaging procedure are controlled, if they are voluntary.
Consciousness is known to be restricted and its time is valuable, only important events get access to it. As measurements of the “channel capacities” of the senses in psychophysical experiments have shown, there is a selection/filtering of the important matters between the information flow in our senses and that in our consciousness. This sophisticated selection also is unconsciously organized and represents an enormous compression of information of at least 104; information flow through the receptors and afferent nerves is at least 105 bit/sec (by order of magnitude), whereas only 10 bit/sec show up in consciousness. The will, however, always takes part, albeit sometimes merely to the degree that it delegates as much as possible to unconscious routines and expert systems of the brain. The unconscious processes therefore do not lessen freedom; on the contrary, they form its primary basis.
Magnetoencephalography (MEG)
In 1981, Hal Weinberg invited me as a distinguished visiting professor to the Simon Fraser University in Greater Vancouver, Canada, and we were the first to record the MEG equivalent of the BP, the “Bereitschaftsfeld, BF” (Deecke, Weinberg Brickett 1982). We investigated the Bereitschaftsfeld accompanying foot movements as did Riita Hari and her group (Hari et al 1983). We also investi-gated slow magnetic fields of the brain preceding speech in Vancouver (Weinberg et al 1983).
Using our own MEG in Vienna, we later investigated the problem of why the SMA (and CMA) activity was not so visible in the MEG (the onset time of the BF was not earlier than 600 msec). The answer is that the two SMAs on the mesial surface of the brain are opposing each other, and if they are both active — which is the case most of the time — their activities cancel each other. We were using two strategies to overcome the problem of cancellation. The first, published by Lang et al (1991), involved investigating a patient with a stroke in one of his SMAs (the right in this case). The patient had only one SMA left, and we were able to record early starting magnetic fields of his left SMA — he performed right-sided movements. The paper’s abstract summarizes this: “Previous studies by magnetoencephalography (MEG) failed to consistently localize the activity of the supplementary motor area (SMA) prior to voluntary movements in healthy human subjects. Based on the assumption that the SMA of either hemisphere is active prior to voluntary movements, the negative findings of previous studies could be explained by the hypothesis that magnetic fields of current dipole sources in the two SMAs may cancel each other. The present MEG study was performed in a patient with a complete vascular lesion of the right SMA. In this case, it was possible to consistently localize a current dipole source in the intact left SMA starting about 1,200 msec prior to the initiation of voluntary movements of the right thumb.”
Our second strategy was to use a multichannel whole scalp MEG instrument on healthy subjects. In Vienna, we had a CTF system with 143 channels and used a sophisticated analysis (two-dipole and three-dipole model in the same subject) to study the Bereitschaftsfield (BF) prior to tapping movements (Erdler et al 2000). The paper’s abstract states: “Despite the fact that the knowledge about the structure and the function of the supplementary motor area (SMA) is steadily increasing, the role of the SMA in the human brain, e.g., the contribution of the SMA to the Bereitschaftspotential, still remains unclear and controversial. The goal of this study was to contribute further to this discussion by taking advantage of the increased spatial information of a whole-scalp MEG system enabling us to record the magnetic equivalent of the Bereitschaftspotential 1, the Bereitschaftsfeld 1 (BF 1) or readiness field 1. Five subjects performed a complex, and one subject a simple, finger-tapping task. It was possible to record the BF 1 for all subjects. The first appearance of the BF 1 was in the range of -1.9 to -1.7 s prior to movement onset, except for the subject performing the simple task (-1 s). Analysis of the development of the magnetic field distribution and the channel waveforms showed the beginning of the Bereitschaftsfeld 2 (BF 2) or readiness field 2 at about -0.5 s prior to movement onset. In the time range of BF 1, dipole source analysis localized the source in the SMA only, whereas dipole source analysis containing also the time range of BF 2 resulted in dipole models, including dipoles in the primary motor area. In summary, with a whole-head MEG system, it was possible for the first time to detect SMA activity in healthy subjects with MEG.”
fMRI – functional Magnetic Resonance Imaging
In 2003, Marjan Jahanshahi and our chairman of the session, Mark Hallett, edited a book titled, “The Bereitschaftspotential — Movement-Related Cortical Potentials” (Jahanshahi M & Hallett M 2003). The closing chapter is written by Deecke and Kornhuber (Deecke & Kornhuber, 2003). The book and chapter for the first time compile all of the evidence that the BP or better “BP-like movement-related activity” can be recorded from the basal ganglia. This cannot be done by EEG or MEG, but requires the fMRI. And not just the routine fMRI but an fMRI improved in such a way that the temporal resolution is high enough to justify the term “event-related fMRI” and in our special case “movement-related fMRI.” This was achieved by Cunnington et al (1999) who showed that it was feasible to record a BP equivalent in the haemodynamic response of the fMRI. Using single-event fMRI in combination with fuzzy clustering analysis, it is possible to analyze the “Bereitschafts BOLD effect” in the form of the haemodynamic response time course. This haemodynamic response resembles the BP or BF but is delayed in time (Cunnington et al 2002).
The movement-related fMRI experiments of Cunnington et al (2002) had great localizatory power and were particularly valuable for mapping the mesial surfaces of the hemispheres, where SMA and CMA are located. In this paper, self-initiated movements were compared with externally triggered movements. In both conditions, the following structures were active: pre-SMA, SMA proper and CMA (cingulate motor area, i.e. part of the anterior cingulate gyrus. The border between pre-SMA and SMA proper is the VAC line (vertical anterior commissure line). Although both pre-SMA and SMA proper are active in self-initiated movements and also in externally triggered ones, there are slight differences: The activity with self-initiated movements is slightly more anterior. It is true that the pre-SMA has somewhat greater involvement in self-initiated movements, but it is clear that it is not the pre-SMA alone that is active (Shibasaki & Hallett 2006) but that both pre-SMA and SMA proper are always activated in voluntary movement.
Using the fMRI, it also was possible to show that the SMA/CMA is active, when a movement is not executed but only imagined. Kasess et al (2008) compared movements that are actually executed with those that are only imagined. They obtained the interesting result that SMA/CMA were active in both imagination and execution, however the motor cortex, M1, was active in executed movements only. In this context, we may report on earlier work using DC-EEG (Uhl et al 1990) and Single Photon Emission Computed Tomography (SPECT) (Goldenberg et al (1989) in mental imagery. We were able to show that the frontal cortex (of which the SMA/CMA form a part) is necessary to bring about the mental imagery (Lang et al 1988; Uhl et al 1990).
Mental imagery is the term in the psychological literature meaning our ability to see something in our mind’s eye. In Uhl et al 1990, we investigated the slow cerebral potentials (DC potentials) accompanying mental imagery. The interesting result was that — with the willful attempt to see something in your mind’s eye (mental imagery) — the frontal cortex was activated first and only thereafter the posterior (sensory) areas of the brain were activated. The strong initial DC negativity of the frontal cortex demonstrated that these frontal areas fulfill an important role in mental imagery: They show that mental imagery is an act of volition, and these frontal areas are needed for the act of bringing about the imagery. In the SPECT study (using the same subjects), analogous results were obtained. These experiments have shown that our “motivational brain” is not only involved when it “exerts itself” in the form of movement or action, but also when it comes to “endogenous acts” (pure mental acts) such as mental imagery, learning with mental rehearsal and thinking. We all know from introspection that the generation of an image in our “mind´s eye” may need considerable effort, and when we increase the effort we achieve a sharper image.
Another important finding of the fMRI studies was that movement-related activity also was recorded from the basal ganglia (Cunnington et al 2002). This finding is important and makes perfect sense in view of the cortico-basal ganglia-thalamocortical loop (cf. Kornhuber, 1974a; Alexander et al (1986); DeLong, 1990). The activity traveling through this loop comes from the SMA/CMA and goes to the M1. On its way, it not only informs the basal ganglia that a movement is about to be initiated but also draws upon the expertise of the basal ganglia as large stores of (over)learned movements and skills. The basal ganglia do the groundwork for the motor cortex M1. In this context, the “chunking hypothesis” of the Hallett group is attractive (Gerloff et al, 1997). These findings are exactly in line with our SMA hypothesis, where we envisage the SMA as a job distributor and supervisor. The SMA organizes sequential tasks in such a way that it breaks down the sequences into handy pieces and reserves the appropriate time slots for their launch. This is what we understand by spatial and temporal coordination. The interesting finding of Cunnington et al (2002, Figure 21) was, however, that activity in the lentiform nucleus (at the junction between the putamen and the external pallidum) was found for self-initiated movements only. For externally triggered movements, there was no evidence of increased activation within the basal ganglia.
A last word about the CMA, the cingulate motor area: Kornhuber and I have reported on this area since the 1990s, but the researcher who worked most intensively on its function is Jun Tanji (Tanji 1994). The cingulate motor areas, located in the banks of the cingulate sulcus, constitute a portion of the cingulate cortex of primates. The rostral cingulate motor area (CMAr) is crucial for reward-based planning of motor selection, whereas the caudal (CMAc) is not (Shima et al 1991). Cunnington recently expanded on this, and investigated concurrent fMRI-EEG and the Bereitschafts-BOLD-effect (cf. his abstract at the end).
To conclude, let me shortly report on the visual hand-tracking experiments of brothers Wilfried and Michael Lang (Lang et al 1983; Deecke et al 1984). In these tracking experiments, designed by them, the temporal course of the moving stimulus was known to the subject while the direction of the moving stimulus (which changes suddenly at a certain time) was unpredictable: The SMA showed antici-patory behavior in that the BP declined ½ sec before the expected change, whereas the directed attention potential (which had its maximum over the parietal area) continued to remain high until 200 msec after the direction change of the stimulus, when the sensory processing was completed. Thus, the frontal lobe, after deciding what to do, delegated further action to posterior cortical areas, which are competent to use visual stimuli and to decide in detail how to perform the tracking task.
From these and similar experiments and from previous results on lesions (Kleist, 1934; Shallice, 1991), Kornhuber developed a theory on the components of volition and their functional local-ization in the frontal lobes (Kornhuber, 1984; Lang et al, 1983, 84; Deecke et al, 1985). One of the stages of volition is planning, and for planning, a working memory is required. The brain is a coop-erative system, but one with strategic organization. There is little doubt that in humans the prefrontal-orbital cortex controls the highest level of planning and decision-making. The frontal lobe is the most humane part of humans. It is due to our frontal lobe that we have the personality we have, equipped with what we call reasoned free will.
This is all laid out in a book that was recently published in English in the U.S., which may be recommended as further reading (Kornhuber & Deecke 2012).
I am ending with the photo taken at the session with Mark Hallett’s camera, and with the abstracts of the other speakers. They are published in Clin. Neurophysiol. and can be found at www.ICCN2014.
S4 Generator Mechanism of BP and Its Clinical Application
Hiroshi Shibasaki, Akio Ikeda (Kyoto/JP)
Since discovery of the slow negative electroencephalographic (EEG) activity preceding self-initiated movement by Kornhuber and Deecke in 1964, various source localization techniques in normal subjects and epicortical recording in epilepsy patients have disclosed the generator mechanisms of each identifiable component of the movement-related cortical potentials (MRCPs). Regarding simple movements, the initial slow segment of BP (early BP) begins about 2 sec before the movement onset in the pre-supplementary motor area (pre-SMA) with no movement site-specificity and in the SMA proper with some somatotopic organization, and shortly thereafter in the lateral premotor cortex bilaterally with relatively clear somatotopy. About 400 ms before the movement onset, the steeper negative slope (late BP) occurs in the contralateral primary motor cortex (M1) and lateral premotor cortex with precise somatotopy. Both early and late BPs are influenced by complexity of the movements while late BP is influenced by discreteness of finger movements. Volitional motor inhibition or muscle relaxation is preceded by BP, which is quite similar to that preceding voluntary muscle contraction. Regarding movements used for daily living such as grasping and reaching, BP starts from the parietal cortex, more predominantly of the dominant hemisphere. BP has been applied for investigating pathophysiology of various movement disorders.
Early BP is smaller in patients with Parkinson disease, probably reflecting the deficient thalamic input to SMA. BP is smaller or even absent in patients with lesions in the dentato-thalamic pathway. Because BP does not occur before involuntary movements, BP is used for detecting the participation of the “voluntary motor system” in the generation of apparently involuntary movements in patients with psychogenic movement disorders.
S5 Movement-Related Desynchronization and Resting State Sensorimotor Networks
Gert Pfurtscheller (Graz/AT)
Preparation for a voluntary movement is not only accompanied by the “Bereitschaftspotential” (BP) and the pre-movement desynchronization (ERD) of central alpha and beta band rhythms but also by a concomitant heart rate (HR) deceleration. The intimate connection between brain and heart was enunciated by Claude Bernard more than 150 years ago (Darwin 1999, pp. 71-72, originally published 1872) and is based on central commands pro-jecting to cardiovascular neurons in the brain stem and modulating the HR.
One interesting question is, why do BP, ERD and HR changes start already some seconds prior to movement onset? It has been documented that the resting state sensorimotor network can oscillate at ~ 0.1 Hz observed in EEG, NIRS-HbO2/Hb and fMRI-BOLD signals (Vanhatalo et al PNAS 2004, Sasai et al Neuroimage 2011). This suggests that the ongoing brain activity can display slow/ultraslow excitability fluctuations in the range of ~10 sec, and voluntary movements are most likely initiated if the excitability in resting state sensorimotor networks reaches a specific threshold. Remarkable is that a close coupling can exist between cerebral and cardiovascular ~0.1 Hz oscillations.
S97 Concurrent fMRI-EEG and the Bereitschafts-BOLD-Effect
*Ross Cunnington
1University of Queensland, School of Psychology & Queensland Brain Institute, Brisbane, Australia
The cortical correlates of voluntary actions precede movement by up to 1-2 sec, as evident in the Bereitschaftspotential. This sustained activity prior to movement is strongly influenced by attention and by arousal levels, showing less activity when actions are relatively unattended and when arousal level is low. While fMRI has revealed key regions that contribute to pre-movement activity, the relationship between activity in these regions and the Bereitschaftspotential is not well understood. By using concurrent EEG and fMRI measurement and single-trial correlation analysis, we find that the cingulate motor area in the mid cingu-late cortex plays a key role in driving sustained activity in the higher motor areas prior to voluntary action. Specifically, we find that trials in which early Bereitschaftspotential activity is large are associated with greater activity in the mid cingulate cortex, and a greater influence of the mid cingulate cortex on sustained activity in the supplementary motor area. This key role of the cingulate motor area in driving sustained activity of the supplementary motor prior to movement can explain how factors such as attention and arousal level also have such a strong influence on early neural activity of the Bereitschaftspotential.
Thanks go to Dr. Volker Deecke, senior lecturer, Centre for Wildlife Conservation, University of Cumbria, UK, for his comments and help with the English.
References:
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally aggregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9: 357-381.
Barrett G, Shibasaki H, Neshige R (1986) Cortical potential shifts preceding voluntary movement are normal in Parkinsonism. Electroenceph Clin Neurophysiol 63:340-348
Cunnington R, Windischberger C, Deecke L, Moser E (1999) The use of single event fMRI and fuzzy clustering analysis to examine haemodynamic response time courses in supplementary motor and primary motor cortical areas. Biomed Technik 44 (Suppl 2): 116-119
Cunnington R, Windischberger C, Deecke L, Moser E (2002) The preparation and execution of self-initiated and externally triggered movement: A study of event-related fMRI. NeuroImage 15: 373-385
Cunnington R, Windischberger C, Deecke L, Moser E (2003) The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. NeuroImage 20: 404-412
Deecke, L., Scheid, P., Kornhuber, HH 1969. “Distribution of readiness potential, pre-motion positivity and motion potential of the human cerebral cortex preceding voluntary finger movements.” Exp. Brain Res. 7, 158-168, criteria met for Citation Classic.
Deecke, L., Grözinger, B., Kornhuber, HH 1976. “Voluntary finger movement in man: Cerebral potentials and theory.” Biol Cybern 23, 99-119, criteria met for Citation Classic.
Deecke, L., Kornhuber, HH 1978. “An electrical sign of participation of the mesial “supplementary” motor cortex in human voluntary finger movement.” Brain Res. 159, 473-476, criteria met for Citation Classic.
Deecke, L; Weinberg, H.; Brickett, P. (1982). Magnetic fields of the human brain accompanying voluntary movement. Bereitschaftsmagnetfeld. Exp. Brain Res. 48: 144–148.
Deecke L, Boschert J, Weinberg H, Brickett P (1983) Magnetic fields of the human brain (Bereitschaftsmagnetfeld) preceding voluntary foot and toe movements. Exp Brain Res 52: 81-86
Deecke L, Eccles JC, Mountcastle (Eds) (1990) From Neuron to Action. An Appraisal of Fundamental and Clinical Research. Springer Publisher. BerlinHeidelbergNew York. xii 677 pp ISBN 3-540-52072-4
Deecke L, Lang W (1990) Movement-related potentials and complex actions: Coordinating role of the supplementary motor area. In: Eccles JC, Creutzfeldt O (Eds): The principles of design and operation of the brain. Pontificiae Academiae Scientiarum Scripta Varia 78, pp 303-336, RomeVatican (1990)
Deecke L, Heise B, Kornhuber HH, Lang M, Lang W (1984) Brain potentials associated with voluntary manual tracking: Bereitschaftspotential, conditioned pre-motion positivity, directed attention potential and relaxation potential. Anticipatory activity of the limbic and frontal cortex In: Karrer R, Cohen J, Tueting P, eds., Ann NY Acad Sci Vol 425: 450-464
Deecke L, Kornhuber HH, Lang W, Lang M, Schreiber H (1985) Timing functions of the frontal cortex in sequential motor- and learning tasks. Hum Neurobiol 4: 143-154.
Deecke L, Kornhuber HH (2003) Human freedom, reasoned will and the brain: The Bereitschaftspotential story. In: M Jahanshahi, M Hallett (Eds) The Bereitschaftspotential, movement-related cortical potentials. Kluwer Academic / Plenum Publishers New York, pp 283-320 ISBN 0-306-47407-7
Deecke L (2012) There are conscious and unconscious agendas in the brain and both are important our will can be conscious as well as unconscious. Brain Sci 2, 405-420
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281-285
Dick JPR, Rothwell JC, Day BL, Cantello R, Buruma O, Gioux M, Benecke R, Berardelli A, Thompson PD, Marsden CD (1989) The Bereitschaftspotential is abnormal in Parkinson‘s disease. Brain 112:233-244
Eccles, J. C. (1973) The understanding of the brain. New York. McGraw-Hill. xv 238 pp
Eccles JC, Creutzfeldt O (Eds) (1990) The principles of design and operation of the brain. Pontificiae Academiae Scientiarum Scripta Varia 78. Rome. Vatican
Erdler M, Beisteiner R, Mayer D, Kaindl T, Edward V, Windischberger C, Lindinger G, Deecke L (2000) Supplementary motor area activation preceding voluntary movement is detectable with a whole scalp magnetoencephalography system. NeuroImage 11: 697-707
Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG (1997) Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain 120: 1587-1602
Goldenberg G, Podreka I, Uhl F, Steiner M, Willmes K, Deecke L (1989) Cerebral correlates of imagining colors, faces and a map – I. SPECT of regional cerebral blood flow. Neuropsychologia 27:1315-1328
Hari R, Antervo A, Katila T, Poutanen T, Seppänen M, Tuomisto T and Varpula T (1983) Cerebral magnetic fields associated with voluntary limb movements in man. Nuovo Cimento 1983, 2D: 484–494.
Jahanshahi M Hallett M (Eds) The Bereitschaftspotential, movement-related cortical potentials. Kluwer Academic / Plenum Publishers New York, viii 334 pp (2003) ISBN 0-306-47407-7
Kant, I. 1785. “Grundlegung zur Metaphysik der Sitten.” Riga: Hartknoch.
Kant, I. 1795. “Zumewigen Frieden.” Königsberg: Nicolovius.
Kasess CH. Windischberger C, Cunnington R, Lanzenberger R, Pezawas L, Moser E (2008) The suppressive influence of SMA on M1 in motor imagery revealed by fMRI and dynamic causal modeling. NeuroImage 40(2) 828-837
Kleist K (1934) Gehirnpathologie. Barth, Leipzig Kornhuber HH, Deecke L (1964) Hirnpotentialänderungen beim Menschen vor und nach Willkürbewegungen, dargestellt mit Magnetbandspeicherung und Rückwärtsanalyse. Pflügers Arch.281: 52.
Kornhuber HH, Deecke L (1965) Hirnpotentialänderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale. Pflügers Arch 284: 1-17 ‘Citation Classic’
Kornhuber, HH 1974. “Cerebral cortex, cerebellum and basal gangalia: an introduction to their motor functions.” In: Schmitt, F.U., Worden, F.G. eds.: The Neurosciences. Third Study Program.CambridgeMass.: MIT Press. 267-280.
Kornhuber HH: (1974) The vestibular and the general motor system. In: HH Kornhuber (Ed.) Handbook of Sensory Physiology VI/2 Vestibular System Part 2 Springer Berlin pp 581-620
Kornhuber HH, Deecke L (Eds): Motivation, motor and sensory processes of the brain: Electrical potentials, behavior and clinical use. Amsterdam, Elsevier, Prog Brain Res Vol 54, 811 pp (1980)
Kornhuber HH, Deecke L, Lang W, Lang M (1989) Will, volitional action, attention and cerebral potentials in man: Bereitschaftspotential, performance-related potentials, directed attention potential, EEG spectrum changes. pp 107-168 in HershbergerWA, ed., Volitional action. Conation and control. Elsevier Science/North Holland.
Kornhuber HH, Deecke L (1990) Readiness for movement — The Bereitschaftspotential story. Citation Classic Commentary. Current Contents Life Sciences 33 (4): 14.
Kornhuber HH, Deecke L (1990) Readiness for movement – The Bereitschaftspotential story. Citation Classic Commentary. Current Contents Clinical Medicine 18 (4): 14
Kornhuber, HH (1978) “Motorische Systeme und Sensomotorische Integration.” In: Stamm, R.A., Zeier, H. eds.: Die Psychologie des 20. Jahrhunderts. Bd. VI. Zürich: Kindler. 750-762.
Kornhuber, HH 1984 “Attention, readiness for action, and the stages of voluntary decision.” Exp. Brain Res. Suppl. 9, 420-429.
Kornhuber, HH 1987. “Handlungsentschluß, Aufmerksamkeit und Lernmotivation im Spiegel menschlicher Hirnpotentiale, mit Bemerkungen zu Wille und Freiheit.” In: Heckhausen, H., Gollwitzer, P.M., Weinert, F.E. eds.: Jenseits des Rubikon: Der Wille in den Humanwissenschaften. Berlin, Heidelberg: Springer. 376-401.
Kornhuber, HH 1988. “The human brain: from dream and cognition to fantasy, will, conscience and freedom.” In: Markowitsch, H.J. ed.: Information processing by the brain. Toronto, Bern, Stuttgart: Hans Huber Publ. 241-258.
Kornhuber, HH 1992. “Gehirn, Wille, Freiheit.” Rev. Metaphys. et Morale. 2. 203-223.
Kornhuber, HH 1993. “Prefrontal cortex and homosapiens: on creativity and reasoned will.” Neu-rol. Psychiat. Brain Res 2, 1-6.
Kornhuber HH, Deecke L (2012) The will and its brain — an appraisal of reasoned free will. University Press of America, Lanham, MD, USA, xvi 116 pp ISBN978-0-7618-5862-1
Lang W, Lang M, Kornhuber A, Deecke L, Kornhuber HH (1983) Human cerebral potentials and visuo-motor learning. Pflügers Arch Eur J Physiol 399: 342-344.
Lang W, Lang M, Heise B, Deecke L, Kornhuber HH (1984) Brain potentials related to voluntary hand tracking, motivation and attention. Hum Neurobiol 3: 235 – 240.
Lang W, Lang M, Podreka I, Steiner M, Uhl F, Suess E, Müller C, Deecke L (1988) DC-potential shifts and regional cerebral blood flow reveal frontal cortex involvement in human visuomotor learning. Exp Brain Res 71: 353-364
Lang W, Cheyne D, Kristeva R, Beisteiner R, Lindinger G, Deecke L (1991) Three-dimensional localization of SMA activity preceding voluntary movement. A study of electric and magnetic fields in a patient with infarction of the right supplementary motor area. Exp Brain Res 87: 688-695
Lang W, Deecke L (1998) Psychophysiologie der Motorik. Chapter 5 in: F Rösler (Ed) Volume 5 Ergebnisse und Anwendungen der Psychophysiologie. Enzyklopädie der Psychologie, Serie I Biologische Psychologie. Hogrefe Göttingen, pp 225-283
Libet B, Gleason CA, Wright EW, Pearl DK (1983) Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). Brain 106: 623-642
Libet B (1990) Cerebral processes that distinguish conscious experience from unconscious mental functions. In: Eccles JC, Creutzfeldt O (Eds): The principles of design and operation of the brain. Pontificiae Academiae Scientiarum Scripta Varia 78, pp 186-202, RomeVatican
Marsden CD, Deecke L, Freund H-J, Hallett M, Passingham RE, Shibasaki H, Tanji J, Wiesendanger M (1996) The functions of the supplementary motor area: Summary of a workshop. Advances in Neurology, Vol. 70: Supplementary Sensorimotor Area, HO Lüders (Ed) pp 477-487
McCallum, WC, Knott JR (1976) The responsive brain. John Wright & Sons Ltd. Bristol 272 pp.
Popper KR, Eccles JC (1977) The self and its brain. An argument for interactionism. Springer International. Berlin, Heidelberg, LondonNew York 597 pp.
Shallice, T., Burgess, P. 1991. “Higher-order cognitive impairments and frontal lobe lesions in man.” In: Levin, H.S., Eisenberg, H.M., Benton, A.L. eds.: Frontal lobe function and dysfunction. New York, Oxford: OxfordUniversity Press, 125-138.
Shibasaki H, Hallett M (2006) What is the Bereitschaftspotential? Clin Neurophysiol 117:2341-2356
Shima K, Aya K, Mushiake H, Tanji J (1991) Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J Neurophysiol 65:188-202
Tanji J (1994) The supplementary motor area in the cerebral cortex. Neurosci Res 19: 251-268
Uhl F, Goldenberg G, Lang W, Lindinger G, Steiner M, Deecke L (1990) Cerebral correlates of imagining colours, faces and a map – II. Negative cortical DC-potentials. Neuropsychologia 28:81-93
Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL (1964) Contingent negative vari-ation: An electric sign of sensori-motor association and expectancy in the human brain. Nature 203: 380-384.
Weinberg H, Brickett P, Deecke L, Boschert J (1983) Slow magnetic fields of the brain preceding movements and speech. Proceedings of the IV Int Workshop on Biomagnetism, Rome Sept, 1982 Il Nuovo Cimento 2: 495-504
Wu T, Kansaku K, Hallett M (2004) How self-initiated memorized movements become automatic: a functional MRI study. J Neurophysiol 91:1690-1698.
Kornhuber HH & Deecke L, The Will and its Brain, University Press of America publisher, 2012.
